SSbD is a foundational principle in the EU Chemicals Strategy for Sustainability (CSS). This objective is in the process of being fully defined, and a new report from the Joint Research Centre is a major contribution to that, see:
Caldeira, C., Farcal, R., Garmendia Aguirre, I., Mancini, L., Tosches, D., Amelio, A., Rasmussen, K., Rauscher, H., Riego Sintes, J. and Sala, S., Safe and sustainable by design chemicals and materials – Framework for the definition of criteria and evaluation procedure for chemicals and materials, EUR 31100 EN, Publications Office of the European Union, Luxembourg, 2022, ISBN 978-92-76-53280-4, doi:10.2760/404991, JRC128591. Publications Office of the European Union JRC128591.
This is a comprehensive report, with a heavy emphasis on “safe”. Undoubtedly it is a major contribution to the definition of SSbD. The authors are not required to be concerned about how innovators get and develop their ideas; there is almost no attention given to that, or indeed to the inherent difficulties in, for example, the development of new science to replace unacceptable practices.
This report is for anyone who wants to understand SSbD, although many would find it far too long for their needs. However, if you want to find out how to balance SSbD with the costs of innovation, you will get no help. There is recognition of the reality that insights into what makes for the desired functionality and Into SSbD evolve during a project.
It is not necessary to list the report’s findings and recommendations here; one thing is clear, that safety comes first in innovation in the views of the authors.
My experience is that innovators do take serious account of regulatory requirements from the start of projects, and of the economic impacts of meeting SSbD criteria. The report does not address the huge cost difference between screening level safety data and full regulatory requirements for registration. This leads to the problem that researchers cannot know the full costs of achieving SSbD before they know whether a project is commercially viable. I believe it is key that functionality and hazard are assessed in parallel from the start even before any experimental work is done.
This need for continuous consideration of functionality and SSbD is mentioned in the report, and it very usefully refers to the source:
Safe-by-Design part II: A strategy for balancing safety and functionality in the different stages of the innovation process. Isabella Tavernaro, Susan Dekkers, Lya G.Soeteman-Hernández, Petra Herbeck-Engel, Cornelle Noorlander, Annette Kraegeloh. NanoImpact, Volume 24, October 2021, 100354.
Although it is about nanotechnology, this is still a helpful diagram from that paper:
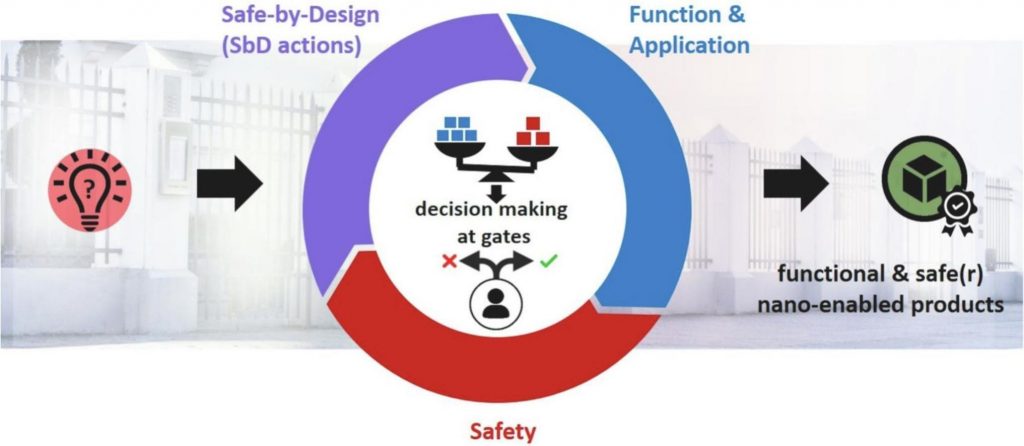
See also other parts of the GCD website!
The JRC report is not intended to answer some of the issues that are raised in my comments. One strategy is to improve chances of commercial success by use of technologies are intrinsically likely to be without hazard, or is bio-based (for example). It is also highly-desirable to find molecular descriptors which describe performance and hazard.
It is also clear that making the most of substances which already have REACH-compliant data sets is a solid strategy e.g. by finding new applications or new formulations.
My conclusion is that the report will not help innovators significantly (they usually know what to do), and the Commission needs to address how to do it with more than funding of research projects.
I would welcome any feedback on my thoughts about SSbD and innovation.